Why Carbon Is Special
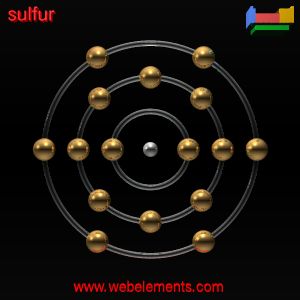
Sulfate Valence Electrons
There are now more than ten million organic compounds known by chemists. Many more undoubtedly exist in nature, and organic chemists are continually creating (synthesizing) new ones. Carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms, and because the carbon atom is just the right, small size to fit in comfortably as parts of very large molecules.
In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Example: Consider the Lewis structure for sulfur tetrafluoride (SF 4) which contains 34 valence electrons. SF 4: 6 + 4(7) = 34. There are four covalent bonds in the skeleton structure for SF 4. Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons.
Having the atomic number 6, every carbon atom has a total of six electrons. Two are in a completed inner orbit, while the other four are valence electrons—outer electrons that are available for forming bonds with other atoms.
The carbon atom's four valence electrons can be shared by other atoms that have electrons to share, thus forming covalent (shared-electron) bonds. They can even be shared by other carbon atoms, which in turn can share electrons with other carbon atoms and so on, forming long strings of carbon atoms, bonded to each other like links in a chain. Silicon (Si), another element in group 14 of the periodic table, also has four valence electrons and can make large molecules called silicones, but its atoms are too large to fit together into as great a variety of molecules as carbon atoms can.
Carbon's ability to form long carbon-to-carbon chains is the first of five reasons that there can be so many different carbon compounds; a molecule that differs by even one atom is, of course, a molecule of a different compound. The second reason for carbon's astounding compound-forming ability is that carbon atoms can bind to each other not only in straight chains, but in complex branchings, like the branches of a tree. They can even join 'head-to-tail' to make rings of carbon atoms. There is practically no limit to the number or complexity of the branches or the number of rings that can be attached to them, and hence no limit to the number of different molecules that can be formed.
The third reason is that carbon atoms can share not only a single electron with another atom to form a single bond, but it can also share two or three electrons, forming a double or triple bond. This makes for a huge number of possible bond combinations at different places, making a huge number of different possible molecules. And a molecule that differs by even one atom or one bond position is a molecule of a different compound.
The fourth reason is that the same collection of atoms and bonds, but in a different geometrical arrangement within the molecule, makes a molecule with a different shape and hence different properties. These different molecules are called isomers.
The fifth reason is that all of the electrons that are not being used to bond carbon atoms together into chains and rings can be used to form bonds with atoms of several other elements. The most common other element is hydrogen, which makes the family of compounds known as hydrocarbons. But nitrogen, oxygen, phosphorus, sulfur, halogens, and several other kinds of atoms can also be attached as part of an organic molecule. There is a huge number of ways in which they can be attached to the carbon-atom branches, and each variation makes a molecule of a different compound. It's just as if moving a Christmas tree ornament from one branch to another created a completely different tree.
Additional topics
Science EncyclopediaScience & Philosophy: Calcium Sulfate to Categorical imperativeCarbon - How Carbon Is Found, Graphite, Diamond, The Chemistry Of Carbon, Why Carbon Is Special - Classes of carbon compounds
Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO2, we can determine the hybridization of atoms.
Sulfur dioxide | SO2
Sulfur dioxide is a colourless inorganic gas and also a toxic gas. It gives a weak acid solution when dissolves in water. This gas is produced due to combustion of petroleum fuel in automobiles and industrial factories.
Lewis structure of SO2
There are two double bonds between sulfur atom and oxygen atoms in SO molecule. Also, a lone pair exists on sulfur atom and each oxygen atom has two lone pairs in SO2 lewis structure.
Hybridization of SO2
All atoms have sp2 hybridization. Each oxygen atom has one sigma bond and two lone pairs. Therefore, oxygen atoms' hybridization should be sp2. For sulfur atom, there are two sigma bonds and one lone pair to make hybridization sp2.
Simple method to determine the hybridization of atoms in covalent compounds
Apply VSEPR theory - Steps of drawing lewis structure of SO2
Following steps are used to draw the lewis structure of SO2. Each step is explained in detail in next sections. If you are a beginner to lewis structure drawing, follow these sections slowly and properly to understand it completely. Look the figures to understand each step.
- Find total number of electrons of the valance shells of sulfur and oxygen atoms
- Total electrons pairs
- Center atom selection
- Put lone pairs on atoms
- Check the stability and minimize charges on atoms by converting lone pairs to bonds until most stable structure is obtained.
Total number of electrons of the valance shells of ethene
Both sulfur and oxygen belongs to the group VIA elements series. Therefore, they have six electrons in their valence shell. To find number of valence electron, these valence electrons of each element should be multiplied with their respective number of atoms in the molecule. Below, That step are done.
- Total valence electrons given by two oxygen atoms = 6 * 2 = 12
- Total valence electrons given by sulfur atom = 6 * 1 = 6

There are no charges in SO2 molecule. Therefore, no addition or reduction of valence electrons due to charges.
- Total valence electrons = 12 + 6 = 18
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Sulfur Valence Electrons Gain Or Lose
Total electron pairs are determined by dividing the number total valence electrons by two. For, SO2 molecule, Total number of pairs of electrons are 9.
Center atom and sketch of ethene molecule
There are some requirements to be the center atom. Having a high valence is a main requirement to be a center atom. For SO2 molecule, sulfur has the highest valence than and oxygen.
Mark lone pairs on atoms
After drawing the sketch, we should start to mark lone pairs on atoms. In the drawn sketch, there are two bonds. between atoms.
- There are already two S-O bonds in the above sketch. Now, there are only seven (9-2 = 7) valence electrons pairs are remaining to draw (as mark lone pairs) the rest of the structure.
- First, mark remaining valence electrons pair as a lone pairs on oxygen atoms (outside atoms). Six valence electron pairs are marked on two oxygen atoms. Now, there are still one valence electron pair is remaining. That is marked on sulfur atom as the lone pair.
- Now, all valence electron pairs are marked as bonds and lone pairs in the sketch.
Charges on atoms
Charges on atoms are important to find the most stable structure. Therefore, we need to find the most stable structure to obtain lewis structure. Therefore, we should try to find charges if there are.
After, marking electron pairs on atoms, we should mark charges of each atom. Each oxygen atoms will get a -1 charge and sulfur atom get a +2 charge. Because SO2 is a neutral molecule, overall charge of the molecule should be zero. The overall charge of the molecule is, (-1) * 2 + (+2) = 0.
Stability of structure and minimize charges on atoms by converting lone pairs to bonds
When there are positive and negative charges on lot of atoms or higher charges (like +2, +3, -2, -3) on atoms in an ion or molecule, that structure is not stable. Therefore, We should try to reduce charges on atoms if it is a possible. In thee above structure, there are charges on oxygen atoms and sulfur atom. Now, we are going to reduce charges on these atoms as below.
- Now, we should try to minimize charges by converting a lone pair or pairs to a bond. So convert a lone pair on a oxygen atom to make a new S-O bond with sulfur atom as the following figure.
- Now there is a double bond between one oxygen atom and sulfur atom. You can see, charges are reduced now in the new structure.
- Because, there are still charges on atoms, we can try to convert a lone pair to a bond. So, convert a lone pair on other oxygen atom to make a bond with sulfur atom. With that, there are no charges on sulfur and oxygen atoms.
- Therfore, that structure should be the lewis structure of SO2
Questions
How many lone pairs sulfur has in SO2
There is only one lone pair on valence shell of sulfur atom.

SO2 lewis structure lone pairs
There are lone pairs on all atoms in SO2. Sulfur atom has one lone pair and each oxygen atom has two lone pairs. Therefore, there are total of five lone pairs on last shells of each atom in SO2.
What are the other similar lewis structures of SO2?
If we consider the shape of SO2, water, nitrogen dioxide, hydrogen sulfide, ozone have similar shape, bent. Also, if number of sigma bonds are is considered, water, nitrogen dioxide, hydrogen sulfide, sulfur dioxide all have two sigma bonds. Both SO2 and ozone has one lone pair on thei center atom.
SnapNDrag Pro is screen capture made ridiculously easy. SnapNDrag started off as a simple app that lets you snap a screenshot with one click and then drag the result off to Mail, Finder or any other app that accepts an image. SnapNDrag Pro 4.4 Torrent is a superb Mac app for organizing and annotating your screenshots. You can simply drag the screenshot into any other application that accepts an image – such as Finder or Mail – or you can rename it and save it to a folder for reference later. Snapndrag pro.
Are there similarities in SO2 and NO2 lewis structures?
Actually, this question is weird one. Ms word 2011 for mac. Rather than discussing similarities, it's good to discuss their differences. In NO2 lewis structure, there is an unpaired electron on nitrogen atom.
What are the similarities and differences in SO2 and SO3 lewis structures?
- Around sulfur atom, summation of lone pair and sigma bonds is three in both SO2 and SO3. Therefore, sulfur atom has sp2 hybridization.
- But, shapes around sulfur atom in SO2 and SO3 are different. In SO2, shape around sulfur atom is bent. But in SO3, shape is trigonal planar.
Related lewis structures
P2O5 lewis structureOH- lewis structureAmmonium ion (NH4+) lewis structureH2CO3 lewis structure