The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
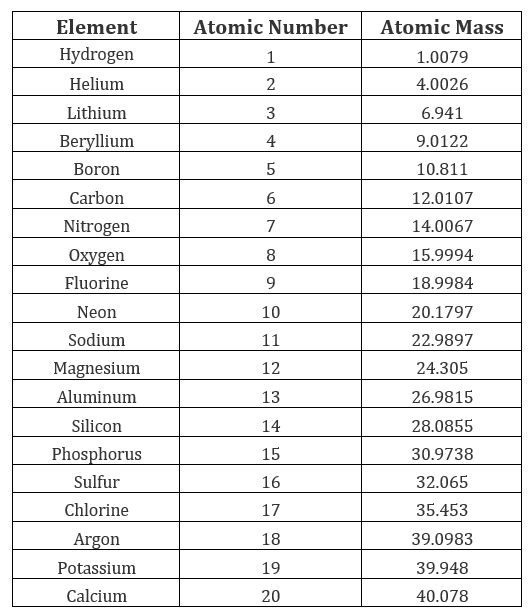
Mass Numbers, Element Symbols, 1st 20 Elements, First Twenty Elements, Atomic Numbers, Periodic Table, Chemistry. Get essential facts about the first 20 elements, all in one convenient place, including the name, atomic number, atomic mass, element symbol, group, and electron configuration. If you need detailed facts about these elements or any of the higher numbered ones, start with the clickable periodic table. Every element on the periodic table is described by two numbers. The larger of the two numbers is the mass number and the smaller number is the atomic number. By the first 20 elements you mean the elements with atomic numbers 1–20 which is the elements hydrogen to calcium 4.7K views. For your grade you need to remember atomic masses of first 20 elements. The atomic mass is double the atomic number of an element. But it is not applicable for all the elements in the periodic table. The atomic masses of elements like He, Li, C, N, O, Ne, Si, S, Cl, Ca are double the atomic number. The atomic masses of elements like Be, B, F, Na, Mg, Al, P, Ar and K are not double the atomic number.
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
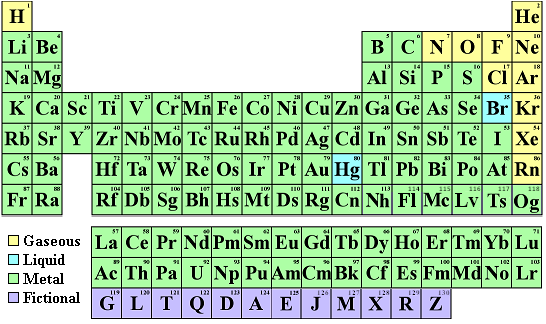
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
ELEMENT : HYDROGEN
SYMBOL: H
ATOMIC NUMBER: 1
ATOMIC MASS: 1 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 1 | – | – | – |
List The Mass Number Of The First 20 Elements
SUBSHELL ELECTRONIC CONFIGURATION: 1s2
VALENCY: 1
GROUP: IA(1) PERIOD:1ST
BLOCK: s
ELEMENT : HELIUM
SYMBOL: He
ATOMIC NUMBER: 2
ATOMIC MASS: 2 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | – | – | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2
VALENCY: 0 (Duplet State)
GROUP: VIIIA(0)or 18 PERIOD:1ST
BLOCK: p
ELEMENT : LITHIUM
SYMBOL: Li
ATOMIC NUMBER: 3
ATOMIC MASS: 7 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 1 | – | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s1
VALENCY: 1
GROUP: IA or 1 PERIOD:1ST
BLOCK: s
ELEMENT : BERYLLIUM
SYMBOL: Be
ATOMIC NUMBER: 4
ATOMIC MASS: 9 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 2 | – | – |
1s2,2s2
VALENCY: 2
GROUP: IIA or 2 PERIOD:2nd
BLOCK: s
ELEMENT : BORON
SYMBOL: B
ATOMIC NUMBER: 5
ATOMIC MASS: 11 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 3 | – | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p1
VALENCY: 3
GROUP: IIIA or 13 PERIOD:2nd
BLOCK: p
ELEMENT : CARBON
SYMBOL: C
ATOMIC NUMBER: 6
ATOMIC MASS: 12 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 4 | – | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p2
VALENCY: 4
GROUP: IVA or 14 PERIOD:2nd
BLOCK: p
ELEMENT : NITROGEN
SYMBOL: N
ATOMIC NUMBER: 7
ATOMIC MASS: 14 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 5 | – | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p3
VALENCY: 3 or 5
GROUP: VA or 15 PERIOD:2nd
BLOCK: p
ELEMENT : OXYGEN
SYMBOL: O
ATOMIC NUMBER: 8
ATOMIC MASS: 16 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 6 | – | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p4
VALENCY: 2
GROUP: VIA or 16 PERIOD:2nd
BLOCK: p
ELEMENT : FLUORINE
SYMBOL: F
ATOMIC NUMBER: 9
ATOMIC MASS: 19 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 7 | – | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p5
VALENCY: 1
GROUP: VIIA or 1 PERIOD:2nd
BLOCK: p
ELEMENT : NEON
SYMBOL: Ne
ATOMIC NUMBER: 10
ATOMIC MASS: 20 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | – | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6
VALENCY: 0 (OCTET STATE)
GROUP: VIIIA(0) or 18 PERIOD:2nd
BLOCK: p
ELEMENT : SODIUM
SYMBOL: Na
ATOMIC NUMBER: 11
ATOMIC MASS: 23 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 1 | – |
1s2,2s22p6, 3s1
VALENCY: 1
How To Remember Mass Number Of First 20 Elements
GROUP: IA(1) PERIOD:3rd
BLOCK: s
ELEMENT : MAGNESIUM
SYMBOL: Mg
ATOMIC NUMBER: 12
ATOMIC MASS: 24 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 2 | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s2
VALENCY: 2
GROUP: IIA(2) PERIOD:3rd
BLOCK: s
ELEMENT : ALUMINIUM
SYMBOL: Al
ATOMIC NUMBER: 13
ATOMIC MASS: 27 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 3 | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s23p1
VALENCY: 3
GROUP: IIIA(13) PERIOD:3rd
BLOCK: p
ELEMENT : SILICON
SYMBOL: Si
ATOMIC NUMBER: 14
ATOMIC MASS: 28 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 4 | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s23p2
VALENCY: 4
GROUP: IVA(14) PERIOD:3rd
BLOCK: p
ELEMENT : PHOSPHORUS
SYMBOL: P
ATOMIC NUMBER: 15
ATOMIC MASS: 30 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 5 | – |
UBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s23p3
VALENCY: 3 or 5
GROUP: VA(15) PERIOD:3rd
BLOCK: p
ELEMENT : SULPHUR
SYMBOL: S
ATOMIC NUMBER: 16
ATOMIC MASS: 32 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 6 | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s23p4
VALENCY: 2
GROUP: VIA(16) PERIOD:3rd
BLOCK: p
ELEMENT : CHLORINE
SYMBOL: Cl
ATOMIC NUMBER: 17
ATOMIC MASS: 34 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 7 | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s23p5
VALENCY: 1
GROUP: VIIA(17) PERIOD:3rd
BLOCK: p
ELEMENT : ARGON
SYMBOL: Ar
ATOMIC NUMBER: 18
ATOMIC MASS: 36 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 8 | – |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s23p6
VALENCY: 0 (OCTET STATE)
GROUP: VIIIA(0 or 18) PERIOD:3rd
BLOCK: p
ELEMENT : POTASSIUM
SYMBOL: K
ATOMIC NUMBER: 19
ATOMIC MASS: 39 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 8 | 1 |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s23p6,4s1
VALENCY: 1
GROUP: IA or 1 PERIOD:4th
Mass Number Of First 20 Elements
BLOCK: s
ELEMENT : CALCIUM
SYMBOL: Ca
ATOMIC NUMBER: 20
ATOMIC MASS: 40 amu
ELECTRONIC CONFIGURATION:
| SHELL | K | L | M | N |
| NO. OF ELECTRONS | 2 | 8 | 8 | 2 |
SUBSHELL ELECTRONIC CONFIGURATION:
1s2,2s22p6, 3s23p6,4s2
VALENCY: 2
GROUP: IIA or 2 PERIOD:4th
BLOCK: s
Compiled by:
Sulaksha Purna Shrestha
Mass Number Of The First 20 Elements
GEMS, Dhapakhel
References:
www.alamy.com (for atomic structures)
www.shutterstock.com (for atomic structures)
www.depositphotos.com (for atomic structures)
Mass Number Of The First 20 Elements
Element cards of first 20 elements
